Patient-derived Models
One of the significant advantages of human cancer organoids and 3D models is their ability to reflect patient-specific responses to treatments. Each patient's cancer is unique, and these models provide a platform to understand and predict how an individual tumour will react to specific therapies. By incorporating patient-derived cells, these models enable researchers to optimise treatment strategies and tailor therapies to individual patients. This personalised approach holds great potential for improving treatment outcomes and advancing personalised cancer medicine.

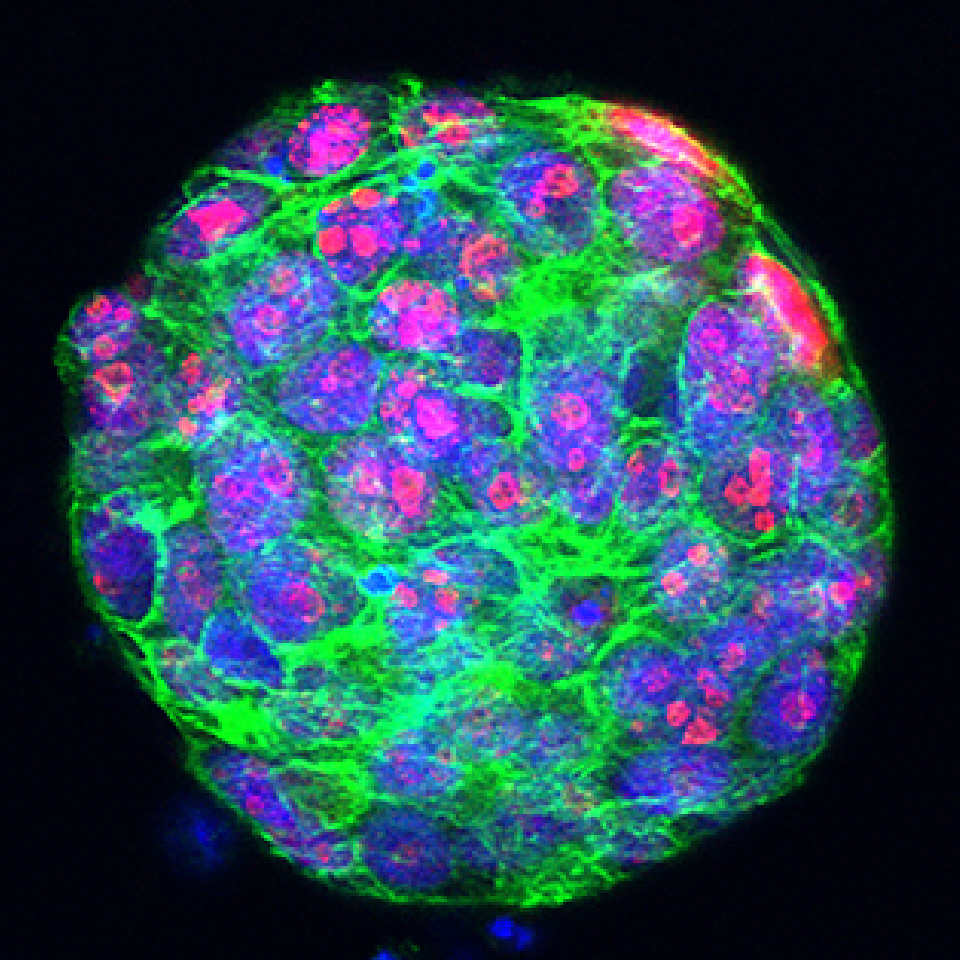
At the forefront of our ambitions, the Centre is dedicated to developing 3D patient-derived models, including organoids, organ-on-chips, and explants. These innovative models establish a direct link between the clinic, the patient, and cutting-edge discovery research, offering a pathway towards the realisation of personalised medicine. Recognising that each patient and every cancer is unique, our goal is to personalise diagnoses, treatments, and disease management, ensuring equal opportunities and the provision of optimal care for everyone facing cancer. Organoids are grown from tumour biopsies or surgical resections, serving as a reservoir of the cancer's biological memory within their genome, epigenome, and proteome. This rich information allows researchers to delve into the precise events that led to the development of this unique cancer and design personalised therapies to combat it. By first testing the efficacy of treatments on organoids, researchers can enhance treatment efficiency, increase the chances of success, and, most importantly, spare patients from unnecessary treatments, potential side effects, and the associated anxiety.
The Centre aims to address human cancer types that pose challenges in deriving and growing as organoids by enhancing methodologies. To achieve this, we strive to optimise growth by controlling their extracellular environment, leveraging our strengths in chemical biology and material science to develop novel media and 3D polymer combinations. Our objective is to establish innovative co-culture methods that faithfully recapitulate the tumour microenvironment, combining tumour cells with stromal and immune cells. Drawing upon our expertise in bioengineering and fluid mechanics, we anticipate replicating tumour environmental features, a prerequisite for gaining deeper insights into tumour biology, physical interactions, forces, and treatment responses. We are committed to developing tools and technologies that enhance the experimental scale and throughput of organoids. This includes establishing drug screening pipelines, bespoke high-content imaging platforms for large-scale 3D imaging, and employing new mathematical and computational approaches for analysis. By integrating these approaches, we bridge the gap between discovery research and clinical applications, facilitating an iterative synergy that enables the design of personalised treatment experiences tailored to each patient's needs, providing them with the best opportunities to overcome the disease.