Super-resolution ultrasound for the monitoring of radiotherapy response in breast cancer
Professor Mengxing Tang (Bioengineering, Imperial College London), Dr Navita Somaiah and Dr Matthew Blackledge (Radiotherapy and Imaging division, The ICR).
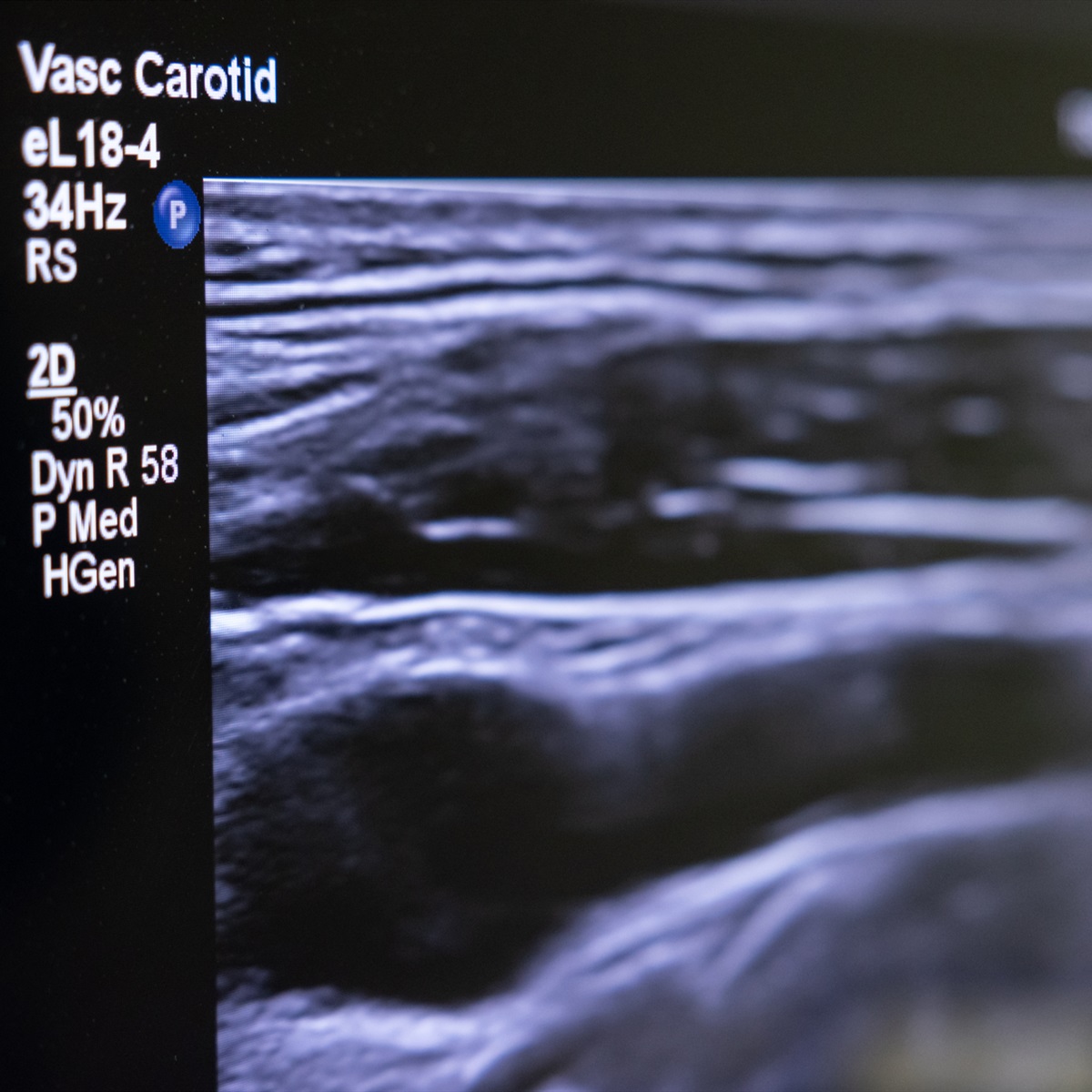
Super-resolution ultrasound (SRUS) is an advanced imaging technique that allows doctors to detect early changes in tumour blood vessels following cancer treatments like radiotherapy. This approach goes beyond simply measuring tumour size, which may take longer to show changes, offering a more immediate and detailed assessment of how well a treatment is working. In a Centre-supported clinical study involving breast cancer patients, SRUS scans were performed multiple times to test their reliability and sensitivity (Morris et al. MedRxiv, 2024). The results showed that SRUS could detect microvascular changes as early as two weeks after radiotherapy, even when tumour size remained unchanged. These early changes were seen in almost all patients within six months, making SRUS a more responsive tool compared to traditional methods. Additionally, when compared to tissue samples, the SRUS findings were consistent with changes in blood vessel density, confirming its accuracy. This research not only highlights the potential of SRUS as a reliable and sensitive method for tracking how tumours respond to treatment, but also offers a kinder and more practical alternative to MRI scans for cancer patients.
Isolating circulating tumour cells to detect metastasis and recurrence
Dr Sam Au (Bioengineering, Imperial College London), Dr Paul Huang (Molecular pathology division, The ICR), Professor Chris Bakal (Cancer Biology division, The ICR).
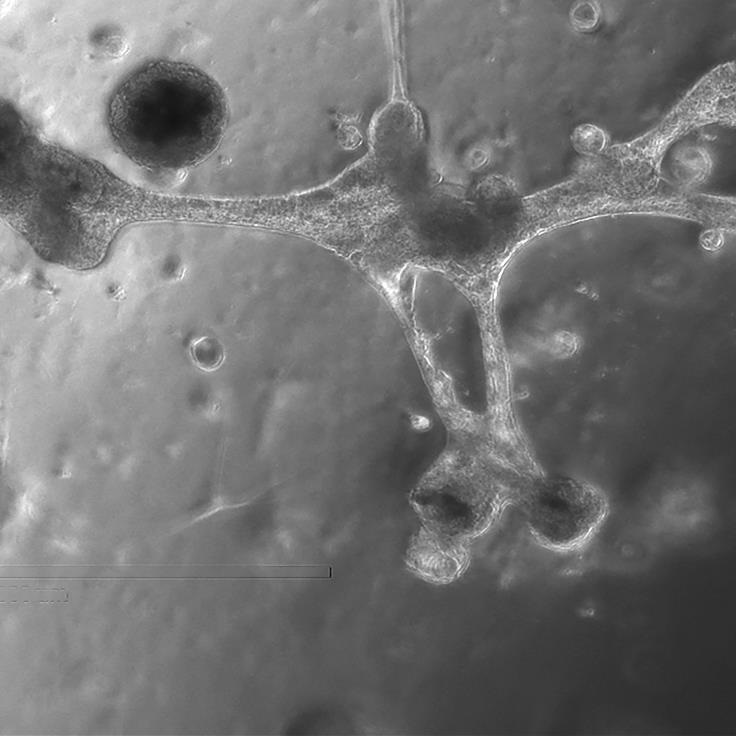
Metastasis occurs when cancer spreads from its original location to other parts of the body, forming new tumours in distant organs. For this to happen, cancer cells must break away from the primary tumour, enter the bloodstream or lymphatic system, and travel to a secondary site where they establish new growths. By isolating these circulating tumour cells (CTCs) from a patient’s blood, researchers can monitor how well treatments are working and predict the risk of recurrence. To achieve this, scientists first need to understand how metastatic cells behave. Researchers supported by the Centre have developed technologies to replicate tiny blood vessels (Kawara et al. Small, 2023), allowing detailed study of how metastatic cells move and interact (Perea Paizal et al. Sci Rep, 2024). Additionally, they have created devices capable of capturing CTCs from blood samples (Au SH, Methods Mol Biol, 2023). These innovations help uncover how metastatic cells influence their environment to form new tumours, including their interactions with blood vessels and immune cells (Vrynas et al. BioRxiv, 2024), advancing strategies to monitor and treat metastasis more effectively.
Putting patients first: Integrating patient-reported outcomes for holistic cancer care
Professor Kalyan Talluri (Business School, Imperial College London), Dr Anna Minchom (Drug Development Unit, The ICR/RM), Dr Christina Yap (CTSU, The ICR).

Patient-reported outcome measures (PROMs) play a vital role in both clinical research and everyday care, offering a direct window into the patient’s experience. By capturing insights into symptoms, quality of life, and treatment impact from the patient’s perspective, PROMs help clinicians better understand and address individual needs. Integrating PROMs with other patient data provides a richer picture of health outcomes and can drive further advancements in cancer research. Recognising the importance of this perspective, the Centre is developing an app tailored to the needs of both patients and clinicians. This innovative tool aims to incorporate patient experiences into research and care, fostering a more holistic and patient-centred approach to cancer management.
A wearable device bringing affectionate touch to cancer care
Professor Helen McNair (Radiotherapy and Imaging, The ICR), Professor Alison McGregor and Professor Fernando Bello (Surgery and Cancer, Imperial College London).
Cancer treatments like radiotherapy and imaging can be highly stressful, often leading to anxiety and even treatment delays. Many patients face these procedures alone due to restrictions, compounding their emotional distress. To address this, researchers have developed a wearable robotic device designed to simulate affectionate touch, offering patients a comforting connection to loved ones during treatment (Hall et al. BMJ Open, 2022). Early trials show that it effectively reduces anxiety and minimises treatment interruptions (Gimson et al. Eur J Cancer Care, 2022). Interruptions in radiotherapy and imaging are a significant concern, with up to 57% of head and neck cancer patients experiencing delays. These disruptions can reduce treatment efficacy and worsen outcomes. While pharmacological solutions for psychological distress are available, they often carry side effects, and non-pharmacological interventions lack standardisation and consistent effectiveness. By offering a simple yet effective way to provide comfort, this innovation promises to enhance patient experiences, reduce anxiety, and ensure uninterrupted treatment, presenting a meaningful advance in the holistic care of cancer patients.

