
Acoustic Cluster Therapy (ACT) is an innovative cancer treatment technology that uses microscopic bubbles combined with ultrasound to enhance drug delivery directly to tumours. This approach offers a non-invasive way to improve the efficacy of chemotherapy by creating precise, targeted delivery systems that can overcome some of the barriers traditional treatments face. By temporarily disrupting the tumour's microenvironment, ACT enables drugs to penetrate more effectively, reducing side effects and increasing their potency. For cancer treatment, this represents a significant leap forward, particularly in addressing hard-to-treat tumours like liver metastases. The technology not only improves drug delivery but also reduces the systemic toxicity associated with chemotherapy, offering patients a more precise and potentially less burdensome treatment option. As studies like ACTIVATE continue to progress, ACT has the potential to transform cancer care by providing safer, more effective therapies tailored to individual tumour characteristics. The ACTIVATE study marks a significant milestone in cancer treatment, demonstrating promising results in early clinical trials for an innovative approach to combat gastrointestinal cancers. The treatment pairs ACT with chemotherapy to target liver metastases. Encouraging outcomes from the phase I trial have led to the study's expansion as a multi-centre clinical trial and further research through reverse translation, exploring how ultrasound and other factors can be optimised to improve drug delivery to tumours.
The intelligent knife for smart surgery
Professor Zoltan Takats (MDR, Imperial College London), Professor the Lord Ara Darzi , Mr Daniel Leff, Professor Sadaf Ghaem-Maghami. Mr James Kinross (Surgery & Cancer, Imperial College London), Professor Maria Kyrgiou (MDR, Imperial College London), Dr George Poulogiannis (Cancer Biology division, The ICR).

The iKnife, or "intelligent knife," is a Centre-supported cutting-edge surgical tool that helps doctors identify cancerous tissue during surgery in real time. It works by analysing the smoke released when tissues are cut with an electrosurgical blade (laser), using mass spectrometry. This allows surgeons to distinguish between healthy and diseased tissue instantly, improving the accuracy of tumour removal and reducing the risk of leaving cancer behind or removing too much healthy tissue. Developed by Professor Takats and his team at Imperial College London (Balog et al. Sci Transl Med, 2013), the iKnife has been successfully tested in various cancers, including breast (St John et al. Breast Cancer Res, 2017), gynaecological (Phelps et al. Br J Cancer, 2018; Tzafetas et al. PNAS, 2020) and colorectal cancers (Alexander et al. Surg Endosc, 2016). This innovation is not only transforming cancer surgeries but also showing promise for broader applications in identifying metabolic conditions (Koundouros et al. Cell, 2020). Ongoing studies are focused on refining its use and integrating it into hospitals worldwide, making surgeries safer and more precise.
Multifunctional fibers for minimally invasive surgery
Dr Burak Temelkuran (MDR, Imperial College London), Professor Zoltan Takats (MDR, Imperial College London), Dr Robbie Murray (Physics, Imperial College London), Professor Vinidh Paleri (Royal Marsden Hospital).

Fiberbots represent an exciting evolution a step ahead of the iKnife technology, paving the way for future surgical tools that are safer, more precise, and highly adaptable. These ultra-thin robotic fibres, under 2 millimetres in diameter, combine cutting-edge advancements in soft robotics, a new type of laser, and optical fibres. Their multifunctionality builds also includes the iKnife with the ability to differentiate tissue types and extends it by offering high-resolution tissue mapping and precise control during minimally invasive surgeries.
Designed for precision, Fiberbots can navigate with movements as fine as 50 micrometres, enabling detailed identification and removal of cancer in hard-to-reach areas. Initial animal studies demonstrated their safety and effectiveness, promising improved surgical accuracy and patient outcomes (Abdelaziz et al. Sci Adv, 2024). With further clinical development underway at the Royal Marsden Hospital, this innovation is a significant step towards next-generation surgical equipment, offering faster recovery times and more patient-centred cancer treatments.
High-performance computing to improve radiotherapy for treating difficult cancers
Professor Uwe Oelfke and Dr Andreas Wetscherek (Radiotherapy and Imaging, The ICR), Professor Wayne Luk (Computing, Imperial College London).
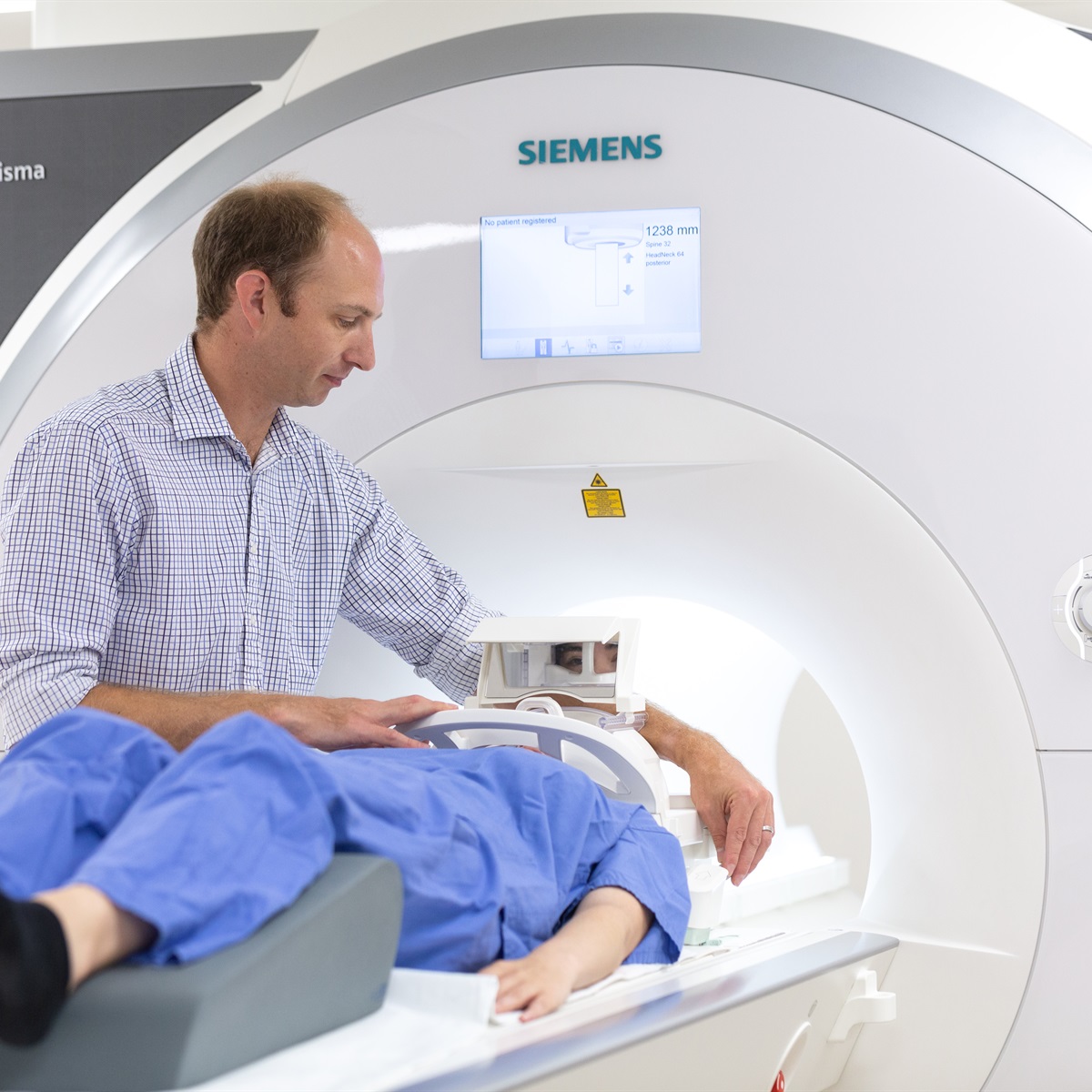
MR-Linac is an advanced medical technology that combines Magnetic Resonance Imaging (MRI) with radiotherapy equipment. This integration allows doctors to obtain detailed images of tumours in real time during radiation treatment, enabling more precise targeting of cancer cells while sparing healthy tissue. However, the real-time nature of MR-Linac is challenging, especially when protecting healthy moving tissue during the intervention such as the lung during breathing. Centre-supported studies have utilised high-performance computing to boost the effectiveness of MR-Linac, particularly for hard-to-treat cancers due to the patient breathing. The team has developed faster and more efficient methods for reconstructing images from 4D-MRI scans (Lecoeur et al. Phys Imaging Radiat Oncol, 2023). By using powerful computing techniques, they have significantly reduced the time needed to process these images, allowing for real-time adjustments to radiotherapy treatments based on the patient’s current anatomy. This precision not only improves the accuracy and effectiveness of the treatment but also minimises side effects.

