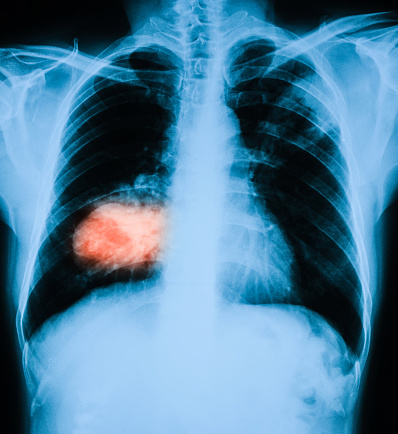
Lung cancer is one of the leading causes of cancer-related deaths worldwide, and early detection is crucial for improving patient outcomes. However, accurately identifying cancerous nodules in the lungs can be challenging, especially when the nodules are small or appear similar to benign growths. Innovative tools that use advanced imaging techniques are helping to bridge this gap, providing doctors with better ways to assess cancer risk and make earlier, more accurate diagnoses. Centre-supported works carried out the development of decision-support tools using radiomics—advanced imaging analysis techniques—to enhance lung cancer diagnosis. These tools improve cancer risk assessment accuracy, surpassing existing clinical models and potentially enabling earlier interventions for large nodules (Hunter et al, eBioMedicine, 2022), and assist radiologists in classifying small lung nodules (Hunter et al. BJC, 2023), a critical step in early lung cancer detection. By integrating advanced image analysis with statistical modeling, the tools outperformed radiologists in predicting malignancy, offering greater diagnostic precision and the opportunity for earlier cancer detection.
Taking a breath - Early detection in pan-alimentary cancers
Professor George Hanna (Surgery & Cancer, Imperial College London), Professor Amanda Cross (Surgery & Cancer, Imperial College London), Professor Peter Buckle (Surgery & Cancer, Imperial College London).

Early gastrointestinal cancers typically have non-specific symptoms that are overlooked, wrongly attributed to common benign conditions, or investigated down the incorrect cancer-site pathway resulting in either missed cases or in delayed diagnosis. Red-flag symptoms characteristic for gastrointestinal cancers are often associated with advanced disease. The current primary investigation for detecting gastrointestinal cancers is endoscopy or cross-sectional imaging, which are invasive, expensive and consume resources. It is not feasible to refer all patients with non-specific gastrointestinal symptoms to have these investigations. The Centre supports the development of a non-invasive breath test to detect gastrointestinal cancers, based on the detection of volatile organic compounds (VOCs) in exhaled breath. A negative test will avoid referring patients while a positive test will prompt urgent referral. The Hanna laboratory discovered VOCs specific for oesophagogastric, pancreatic, liver and colorectal cancers to differentiate between cancer patients and positive controls in 4000 patients. They also established a high-throughput breath analysis platform with excellent limits of detection, quantification, reproducibility, and analytical recovery of VOCs.
Key recent publications: Markar et al. JAMA Oncol., 2018; Woodfield et al. BMJ, 2021, Belluomo et al. Nat Protoc, 2021; Woodfield et al. Gastroenterology, 2022.
New generation of medical devices for the detection of prostate cancer
Dr Sylvain Ladame (Bioengineering, Imperial College London), Professor Charlotte Bevan (Surgery & Cancer, Imperial College London), Professor Joshua Edel (Chemistry, Imperial College London), Professor Pantelis Georgiou (EEE, Imperial College London), Dr Melpomeni Kalofonou (EEE, Imperial College London).
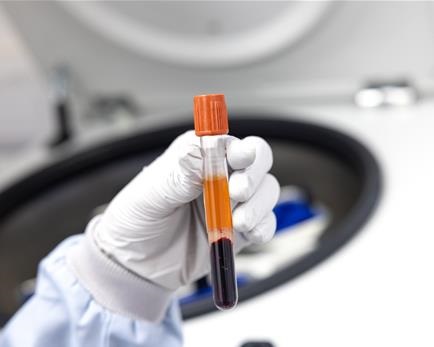
Our researchers are developing innovative, minimally invasive technologies to improve prostate cancer detection and monitoring using biomarkers like microRNAs (miRNAs) and mRNA found in blood samples. Our Centre supported the creation of a low-cost blood test that uses engineered molecules to detect miRNA biomarkers associated with prostate cancer. This enzyme-free, portable test is highly accurate, requires minimal processing, and could be used for widespread cancer screening (Metcalf et al. Anal Chem, 2016). We also helped developing another method using electro-optical nanopore and engineered molecular probes to detect multiple miRNA types directly in very small blood samples. This approach is highly sensitive and specific, enabling precise diagnosis and monitoring of prostate cancer activity and remission (Cai et al. Nat Commun, 2021). We also invested in electronical solution called Lab-on-chip to detect mRNA linked to prostate cancer in the blood. For advanced prostate cancer, a portable device uses chemical sensors to identify mRNA biomarkers linked to drug resistance. This quick, point-of-care test translates biological signals into electrical outputs, offering real-time insights into treatment response (Broomfield et al. ACS Sensors, 2022; Broomfield et al. IEEE Sens Lett, 2023).
These technologies aim to transform prostate cancer care by providing accurate, cost-effective tools for early diagnosis, personalised treatment, and ongoing monitoring.
Innovative imaging technique to improve immunotherapy for glioblastoma patients
Dr Gabriela Kramer-Marek (Radiotherapy and Imaging, The ICR), Dr Phillip Miller (Chemistry, Imperial College London).
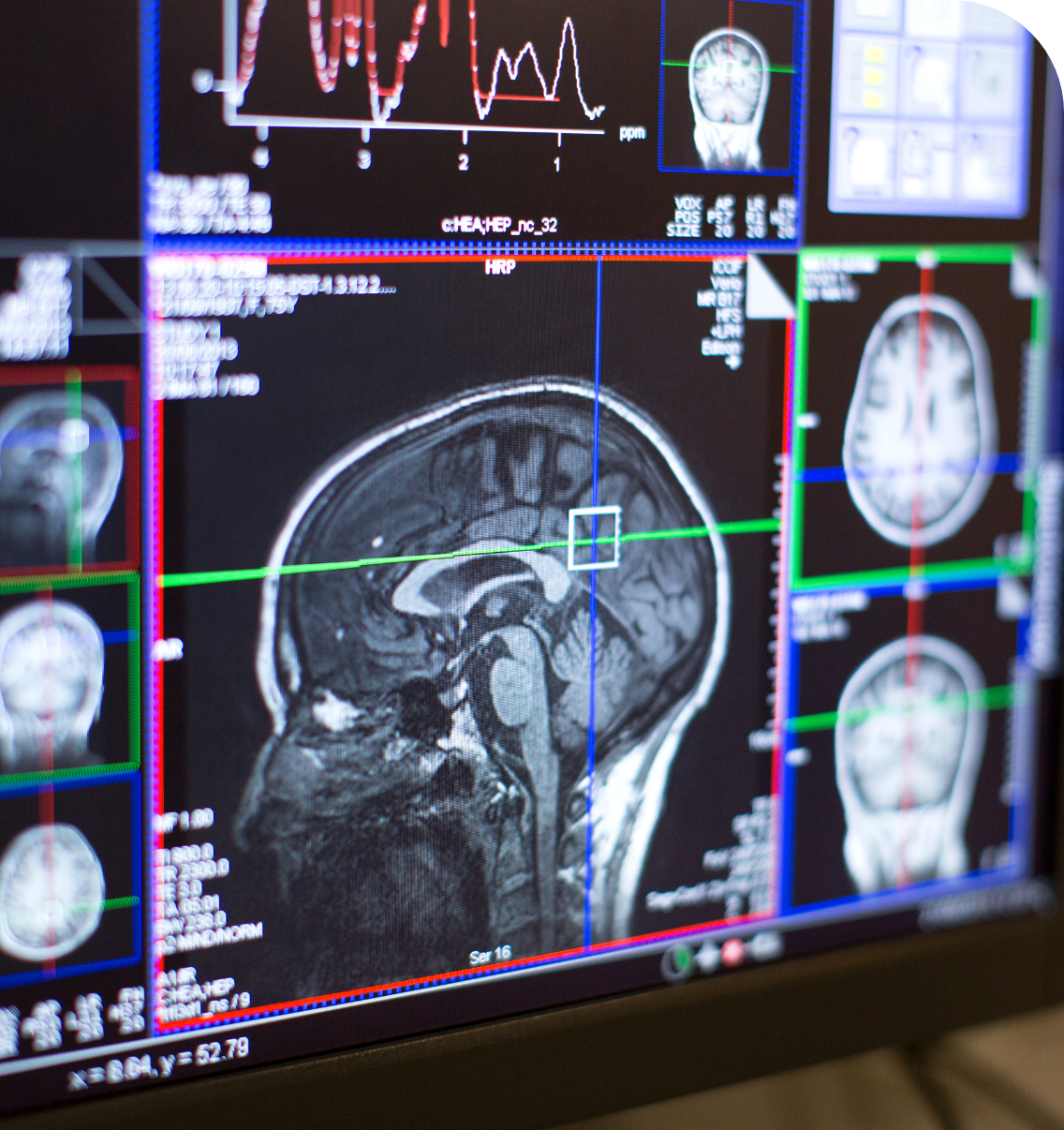
A new imaging method developed by researchers, supported by the Centre, could transform treatment for glioblastoma, an aggressive brain tumour. This technique uses a special tracer to detect levels of PD-L1, a protein that helps predict how well a patient might respond to immunotherapy (Sharma et al. Cancers, 2023). Importantly, it works without the need for invasive biopsies. Early trials in humans showed that the tracer can successfully track PD-L1 levels and monitor how the immune system responds to treatments like pembrolizumab. In some cases, this approach has even helped to slow down the progression of cancer (Dar et al. Neuro Oncol, 2024).
This breakthrough is part of a growing area of medicine called theranostics, which combines diagnosis and therapy to provide highly personalised treatments. By helping doctors match the right treatment to the right patient and supporting their participation in clinical trials, this innovation brings new hope for those battling glioblastoma.

